Date:
It is likely that in the not-distant future wounds will heal faster with the help of an electrical pulse that promotes rapid cell growth. The same type of pulse may be used for more efficient and effective delivery of drugs to fight disease. Such treatments rely on a process known as “electroporation,” in which an electrical field is applied to cells to increase the permeability of the cell membrane. Already electroporation is being used experimentally to deliver chemotherapy into cancerous cells, but such treatments are in their infancy and involve a great deal of trial and error.
Mechanical engineering professor Frederic Gibou and a team of UC Santa Barbara colleagues have developed the first computational models to study electroporation at the tissue scale. Their research, funded by the U.S. Army Research Office’s (ARO) Combat Capabilities Development Command Army Research Lab, is published in the Journal of Computational Physics.
Said Gibou, chair of the Department of Mechanical Engineering, “This is an exciting but mainly unexplored area that stems from a deeper discussion at the frontier of developmental biology, namely how electricity influences morphogenesis, the biological process that causes an organism to develop its shape.”
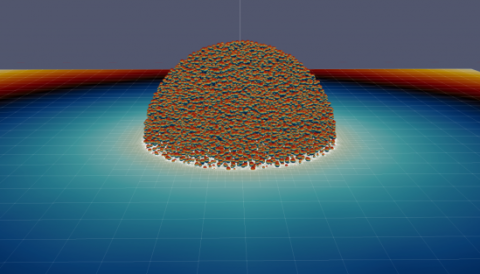
Until now, simulations of the effects of electroporation have been conducted on, at most, a few individual cells at a time. But an entire collective system of cells functions much differently than simply the sum of its cellular parts. Not only is it currently not possible to observe the cells as a system in situ, but variables such as environmental temperature and pressure, as well as the strength, duration and pattern of an introduced electrical field, affect the behavior not only of individual cells but, more importantly, of the system as a whole.
“When you consider a large number of cells together, the aggregate exhibits novel coherent behaviors,” said Pouria Mistani, a Ph.D. student in Gibou’s group and first author of the paper. “It is this emergent phenomenon — novel behaviors that emerge from the coupling of many individual elements —that is crucial for developing effective theories at the tissue-scale.”
What has been missing to arrive at such a system-scale understanding are the computational algorithms that Gibou’s group created. “There is quite a lot of computational science that goes into the design of algorithms that can consider tens of thousands of well-resolved cells,” Gibou noted. “We devised the parallel algorithms for describing the physics at the cell membranes and then implemented them into a super computer at the Texas Advanced Computing Center. The simulations gave us the data from the summation of the individual cells, and that data gave us access to the emergent phenomenon of the cluster, which we can now mine to develop a theory.”
To view the complete story, please read On the Pulse by James Badham published in The Current.
Further reading on this work can be found below.
National Supercomputing Facilities:
Specialized HPC Magazines:
Funding Agencies:
- United States Army and U.S. Army Research Lab
Popular Science Magazines:



